
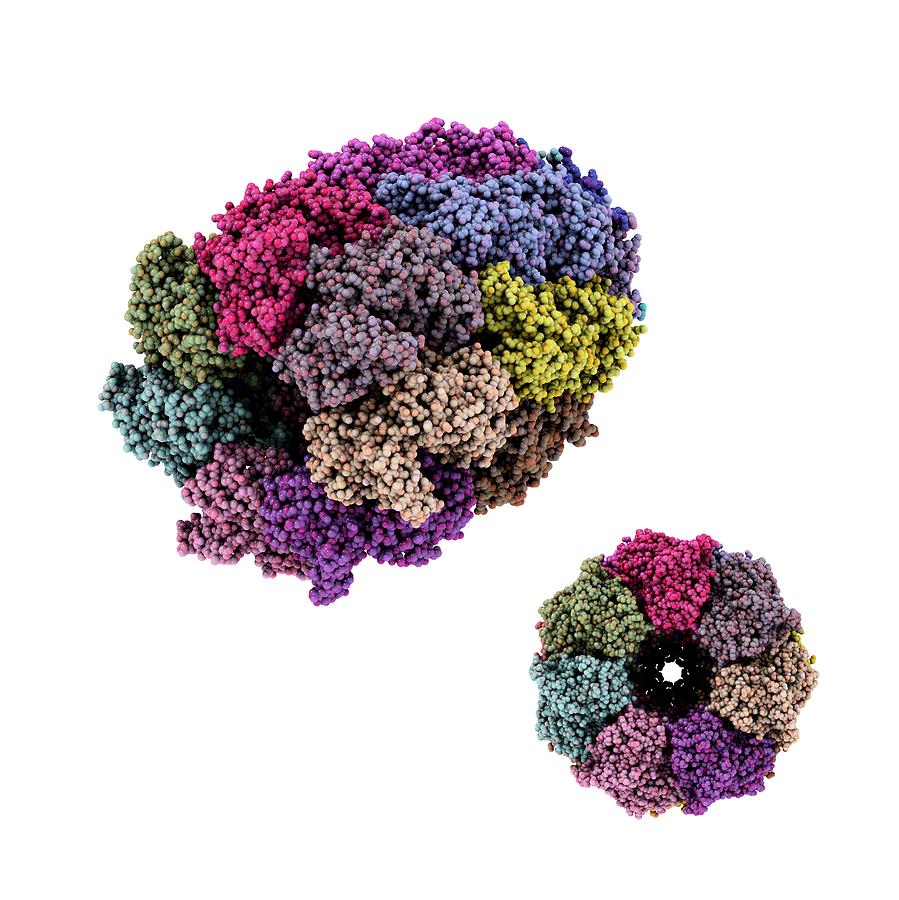
The researchers dubbed GroEL and the Rubisco-binding protein chaperonins, thus defining a subset of the chaperone family, whose members were thought to encourage macromolecular assembly by helping components avoid improper liaisons with themselves and others. In 1988, these observations coalesced when Ellis and Georgopoulos sequenced the GroEL and Rubisco-binding protein genes and established their strong similarity. He proposed that this Rubisco-binding protein promotes assembly of the enzyme. John Ellis (University of Warwick) identified a chloroplast factor that physically interacts with newly made subunits of Rubisco, a key metabolic enzyme. He subsequently tracked the defects to neighboring genes, groEL and groES. In 1972, Costa Georgopoulos (then at Stanford University) discovered that viral proteins fail to congregate into normal “head” structures within a strain of Escherichia coli that harbors particular genetic flaws. The idea that some proteins need help to assemble had emerged from work on bacteria and chloroplasts. The evidence strongly favored a block in folding, but assembly requires that a protein has achieved its proper form, so the experiments could not absolutely distinguish these possibilities. This result suggested that the deviant yeast strain carries a damaged version of a component that normally facilitates protein folding or subsequent events, such as multi-molecular assembly, necessary for protein activity. One of the mutants could transport proteins into mitochondria and clip them to the correct size - but the proteins lacked function, the team reported in 1989. He isolated yeast strains that fail to perform this essential task under certain conditions and then teamed up with Hartl (then at the University of Munich) to analyze the cells’ misbehavior. Horwich sought cellular machinery that participates in the mitochondrial-import process. Once inside, the amino acid sequences that targeted them to the mitochondria are removed, the proteins refold and, in many cases, assemble into multi-part structures before gaining enzymatic activity. Gottfried Schatz (University of Basel) and Walter Neupert (University of Munich) had shown that proteins are imported into mitochondria in a stretched-out state. In the late 1980s, Hartl and Horwich were studying how proteins that are made in the cytoplasm enter mitochondria. Finally, new proteins in living creatures face a challenge that denatured, full-length proteins in a test tube circumvent: Because they gain a single amino acid at a time, portions of the growing chain can potentially stick to one another before the entire molecule is available to fold properly. Furthermore, inside cells, protein concentrations are orders of magnitude higher than those that Anfinsen used, and as concentration rises, so does the risk of aggregation. However, larger, more complicated proteins than those studied by Anfinsen aren’t as self-sufficient and obliging. Scientists assumed that newly synthesized proteins in cells fold unaided and without energy input, as they can in the test tube. The impact of Anfinsen’s discovery was huge. The protein regained enzymatic activity without assistance, thus establishing that a protein can do its own origami. He added chemicals that unfold - or denature - a small protein and then removed these agents. In the late 1950s and early 1960s, Christian Anfinsen (National Institutes of Health) showed that the amino acid sequence of a protein supplies the information it needs to assume its final form. Horwich and Hartl discovered that a special apparatus encases an unfolded protein and spurs folding by harnessing the energy of ATP, the small molecule that drives reactions inside cells. As proteins take shape, they bury these hydrophobic parts and expose hydrophilic, or water-loving, areas. Greasy regions of new proteins, however, can grab one another and create useless globs.
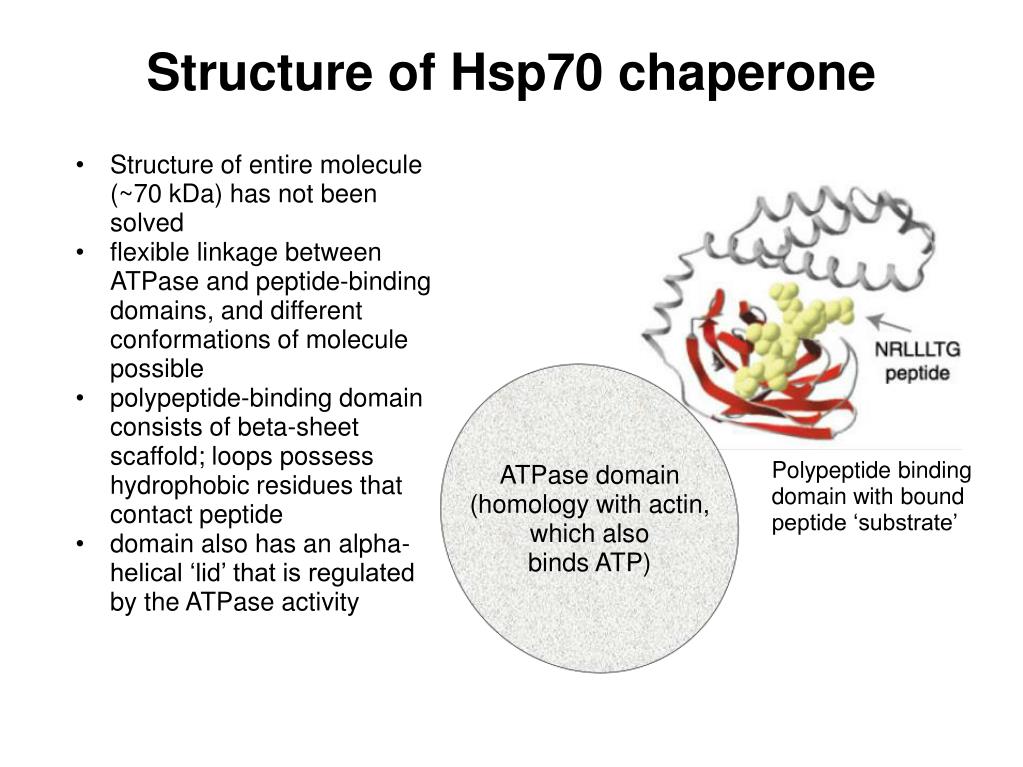
Protein folding is a vital process, as it converts linear amino acid chains into the three-dimensional forms that bestow the molecules’ unique activities. This previously unexplored realm holds enormous importance for basic biology and biomedicine. Horwich (Yale University School of Medicine) toppled traditional notions of how proteins fold inside cells and established new principles that operate from microbes to humans. With this work, Franz-Ulrich Hartl (Max Planck Institute of Biochemistry, Martinsried) and Arthur L. The 2011 Albert Lasker Basic Medical Research Award honors two scientists for their discoveries concerning the cell’s protein-folding machinery, exemplified by cage-like structures that convert newly made proteins into their biologically active forms.


 0 kommentar(er)
0 kommentar(er)
